Roadmap for an Integrated Life Sciences Ecosystem In Ontario
LSO - Life Sciences Ontario - prepared an in-depth and informative report on the importance of the life science sector in Ontario. TIAP was one of the endorsing organizations. The…
LSO - Life Sciences Ontario - prepared an in-depth and informative report on the importance of the life science sector in Ontario. TIAP was one of the endorsing organizations. The…

MaRS Innovation’s “model solves the two weakest points in tech transfer: the lack of dealflow and the ability to match public funding,” writes Thierry Heles in, “MaRS Innovation: A Unique Model for Tech Transfer,” for Global University Venturing.
This feature was also covered in Techopia.
The article, which includes an interview with Dr. Rafi Hofstein, president and CEO of MaRS Innovation, was published September 14, 2015.
Here’s an excerpt exploring the range of MI’s portfolio and Hofstein’s strategy for addressing technologies emerging in new areas:
In the beginning, Mars received primarily discoveries in the medical sector, but the balance has since shifted to 60% medically-oriented research and 40% for other areas. The medically-oriented discoveries, Hofstein elaborated, are a diverse set of technologies and include everything from drug development and molecular diagnostics to medical devices and healthcare IT.
The remaining 40% meanwhile cover “a smörgåsbord all the way from alternative energies and solar energy, and water reclamation to all sorts of mobile apps”.
 TORONTO and SAN DIEGO (Feb. 26, 2015) — Triphase Accelerator Corporation has entered into an academic center collaboration with Sunnybrook Research Institute (SRI), the research arm of Sunnybrook Health Sciences Centre, a MaRS Innovation member institution. MaRS Innovation is also a Triphase investor.
TORONTO and SAN DIEGO (Feb. 26, 2015) — Triphase Accelerator Corporation has entered into an academic center collaboration with Sunnybrook Research Institute (SRI), the research arm of Sunnybrook Health Sciences Centre, a MaRS Innovation member institution. MaRS Innovation is also a Triphase investor.
 Under the agreement, SRI will assist in the development of Triphase’s novel, first-in-class, fully human bi-specific antibody TRPH 011 and evaluate the role of bifunctional targeting of VEGFR-2 and TIE 2 receptors in cancer. TRPH 011 binds and neutralizes VEGFR-2/KDR and TIE 2 receptors simultaneously, resulting in sustained inhibition of tumor growth and angiogenesis (the formation of new blood vessels from pre-existing vessels and a fundamental step in the transition of tumors from a benign to a malignant state).
Under the agreement, SRI will assist in the development of Triphase’s novel, first-in-class, fully human bi-specific antibody TRPH 011 and evaluate the role of bifunctional targeting of VEGFR-2 and TIE 2 receptors in cancer. TRPH 011 binds and neutralizes VEGFR-2/KDR and TIE 2 receptors simultaneously, resulting in sustained inhibition of tumor growth and angiogenesis (the formation of new blood vessels from pre-existing vessels and a fundamental step in the transition of tumors from a benign to a malignant state).
Under the terms of the agreement, Triphase will provide funding to the laboratory of Dr. Robert S. Kerbel, senior scientist in the Biological Sciences Platform at SRI. Dr. Kerbel and his colleagues will evaluate TRPH 011 in preclinical pharmacology models. Triphase will use the findings to advance the TRPH 011 program toward an Investigational New Drug (IND) filing.

Biotechnology Focus, a compendium of the Canadian life sciences industry, has published a guest column by MaRS Innovation President & CEO, Dr. Raphael Hofstein.
The article explores the role life sciences assets, financing and talented management–the three Ms–must play in revitalizing Canada’s biotechnology sector:
At the close of the 20th century, Canada was perceived as a key contributor to the success of the global biotech voyage.
You know what happened next: the mechanisms to fund early ventures collapsed together with the collapse of the Canadian venture capital industry Finding suitable investment for early-stage technologies became incredibly challenging. Facing a dearth of opportunity, talented management sailed for other harbors.
It’s satisfying that on the eve of the 2014 BIO Convention, some indicators suggest to me that we are witnessing a rebound. But to accelerate our pace while holding this bearing, Canada needs to address certain strategic elements.
At MaRS Innovation, we call them the three Ms: merchandize, management and money.
TORONTO, ON (May 2, 2014) – MaRS Innovation congratulates the government’s deepened commitment to support the life sciences through research and innovation funding. In particular, the new $30 million Life Sciences Seed Venture Capital Fund will create a partnership between the Province of Ontario, the private sector and hospital foundations to finance Ontario-based life sciences companies.

“As co-designers of this venture capital fund and one of its many champions, MaRS Innovation welcomes this news,” says Dr. Raphael (Rafi) Hofstein, president and CEO. “We look forward to the strong collaborations it will foster with our colleagues in the Government of Ontario, private industry and the hospital community.”
“By their nature and the need for regulation, life sciences companies take considerable time to mature products and their underlying technologies. Expanding the funding available during this critical stage through this unique public-private partnership will give more Ontario start-ups emerging with disruptive technologies from the province’s academic institutions the financing they need to succeed and thrive,” says Hofstein.
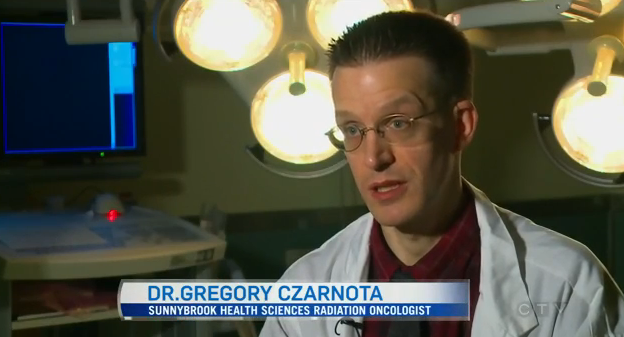
CTV National News featured WaveCheck’s crowdfunding campaign on December 15 in a report by Avis Favaro. The report included an interview with MaRS Innovation’s President and CEO, Dr. Raphael Hofstein (at the 1:37 mark).
William Tran, a researcher associated with the project at Sunnybrook Health Sciences Centre, was also interviewed on Canada AM on December 16.
WaveCheck, which closed its campaign December 4, was invented by Dr. Gregory Czarnota of Sunnybrook Health Sciences Centre and Prof. Michael C. Kolios of Ryerson University. WaveCheck uses ultrasound technology to show people with breast cancer if their chemotherapy is working within weeks.
While the Indiegogo campaign has concluded, Sunnybrook Foundation is now accepting donations flagged “WaveCheck” on behalf of the researchers through its website.
At campaign close, WaveCheck ranked in the top 0.005 per cent of health-related campaigns on Indiegogo, and was covered by CBC television and Metro Morning, the Toronto Star, Sing-Tao and MedCity News.
 The Cellax technology was profiled in a recent issue of SciBX (subscription necessary). MaRS Innovation is mentioned in the article as the technology’s commercialization agent.
The Cellax technology was profiled in a recent issue of SciBX (subscription necessary). MaRS Innovation is mentioned in the article as the technology’s commercialization agent.
Here’s an excerpt:
“Ontario Institute for Cancer Research scientists have developed glycopolymer-conjugated docetaxel nanoparticles that outperform Abraxane in mouse models of breast cancer. The Ontario Institute for Cancer Research (OICR) is backing the program with $1.5 million to take it to the clinic. The expectation is that the product’s ability to target the tumor stroma rather than the tumor itself will differentiate it from Abraxane and other chemotherapeutic formulations.”

“It’s one thing to invent a machine that prints skin, but it’s a whole other challenge to bring what seems like the domain of mad science to mass production,” Matthew Braga wrote in “Looking for ways to get ‘skin’ in the game,” published in the Financial Post on July 15.
The article focuses on MaRS Innovation’s (MI) and the Innovations and Partnerships Office’s (University of Toronto) joint efforts to commercialize the bio printer, a “prototype 3D printer that, instead of extruding layers of plastic and other inorganic materials into physical shapes, builds layer upon layer of cell-laden tissue, a process that could lead to the cheap, rapid production of human skin.”
Braga’s article was syndicated in the Regina Leader Post, the Saskatoon Star-Phoenix, and the Vancouver Sun, among other Canadian publications.
Biotechnology Focus, a compendium of the Canadian life sciences industry, has published a guest column by MaRS Innovation President & CEO, Raphael Hofstein. The article explores the role of catalysis…
Every six weeks, MaRS Innovation’s marketing and communications manager writes a guest post for the MaRS Discovery District blog profiling MI’s activities or one of our start-up companies. You can read the original post on the MaRS blog.

Nearly 14,000 delegates—representing over 1,100 biotechnology companies, academic institutions, state biotechnology centres and related organizations across the United States and more than 60 countries—attended the 2013 BIO International Convention from April 22 to 26, 2013.
The event drew biotechnologists, pharmaceutical industry executives and life sciences researchers, along with sector-based organizations and associations, to Chicago.
According to a press release issued by the conference organizers, BIO 2013 offered “a record number of partnering meetings and panel sessions on the latest science, policy issues and business opportunities and challenges facing the biotechnology industry.”