BlueDot, formerly BioDiaspora Inc., secures Series A with Horizons Ventures
Toronto-based commercial arm of BioDiaspora research program tracks global spread of infectious diseases in real-time; fourth MI company to reach Series A
 TORONTO (Dec. 2, 2014) — BlueDot, a Toronto-based social benefit corporation founded by Dr. Kamran Khan, an infectious disease physician and scientist, tracks and predicts the global spread of infectious diseases.
TORONTO (Dec. 2, 2014) — BlueDot, a Toronto-based social benefit corporation founded by Dr. Kamran Khan, an infectious disease physician and scientist, tracks and predicts the global spread of infectious diseases.
Spun off from St. Michael’s Hospital in partnership with MaRS Innovation (and formerly known as BioDiaspora Inc.), BlueDot, has secured a Series A venture capital funding from Horizons Ventures. Funded by Sir Li Ka-shing, Horizons invests in what they call “game-changing disruptive tech,” and has a proven track record in making early-stage investments (i.e., Facebook, Skype, Waze, Siri and Spotify).
TechVibes and MedCity News covered BlueDot’s Series A announcement, as did PE Hub and BetaKit. Read the BlueDot press release here.
The company is the fourth in MaRS Innovation’s portfolio to reach Series A. MaRS Innovation provided $400,000 in seed funding and worked with BlueDot and St. Michael’s to incorporate the company and develop its initial business strategy, intellectual property protection strategy and go-to-market plan. The Ontario Centres of Excellence also provided $140,000 in commercialization grants that helped BlueDot get off the ground.
BlueDot is the commercial arm of Dr. Khan’s academic research program called BioDiaspora, which was developed at the Li Ka Shing Knowledge Institute of St. Michael’s. BioDiaspora models how infectious diseases can spread and impact populations globally by analyzing big data such as the annual movements of more than 3 billion travelers on commercial flights; human, animal and insect population data; climate data from satellites; and news reports of disease outbreaks. The program was inspired by the Toronto’s SARS crisis in 2003 and its capabilities scientifically validated in prestigious academic journals such as the Lancet and the New England Journal of Medicine.
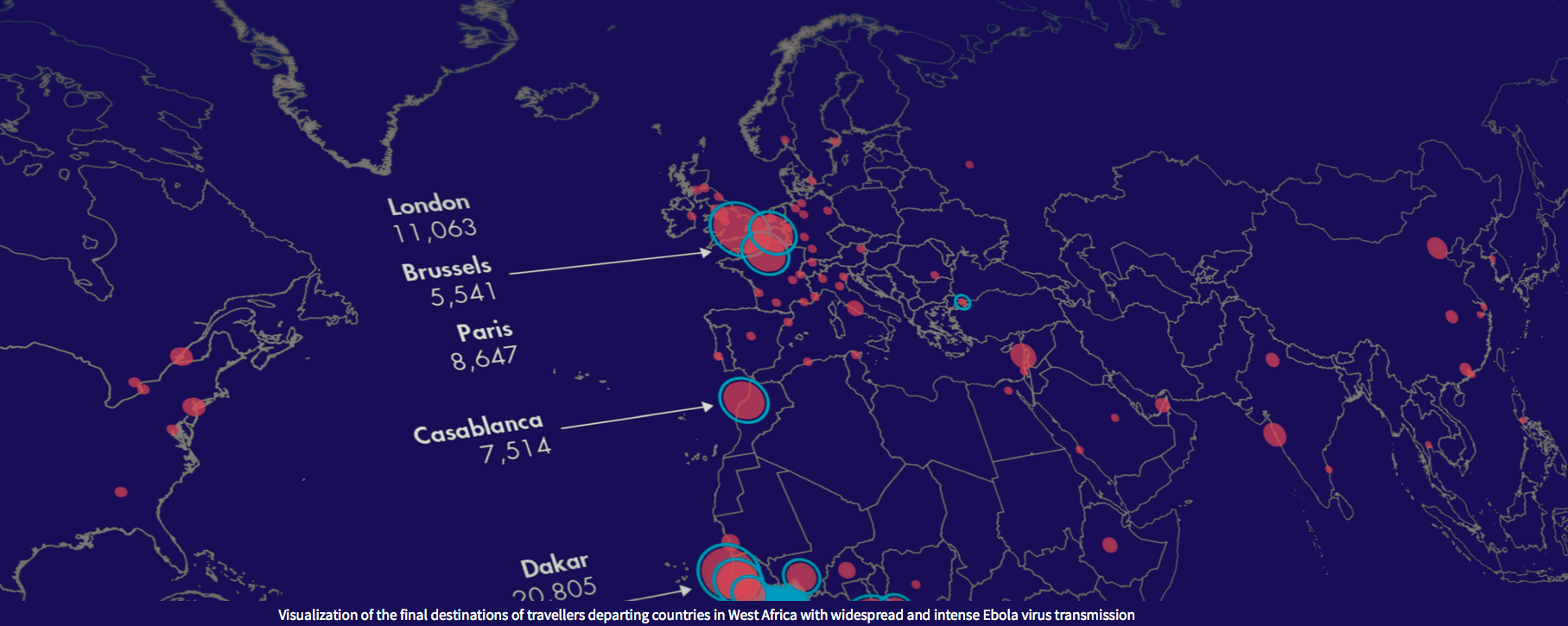
During its development, BlueDot’s platform technology was used by numerous international agencies, including the U.S. Centers for Disease Control, the World Health Organization, the European Centre for Disease Prevention and Control and the Public Health Agency of Canada to evaluate emerging infectious disease threats, including those during global mass gatherings such as the Olympics and the hajj.






