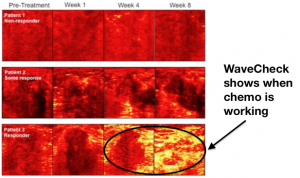
Triphase Accelerator announces new cancer collaboration with Sunnybrook Research Institute
 TORONTO and SAN DIEGO (Feb. 26, 2015) — Triphase Accelerator Corporation has entered into an academic center collaboration with Sunnybrook Research Institute (SRI), the research arm of Sunnybrook Health Sciences Centre, a MaRS Innovation member institution. MaRS Innovation is also a Triphase investor.
TORONTO and SAN DIEGO (Feb. 26, 2015) — Triphase Accelerator Corporation has entered into an academic center collaboration with Sunnybrook Research Institute (SRI), the research arm of Sunnybrook Health Sciences Centre, a MaRS Innovation member institution. MaRS Innovation is also a Triphase investor.
 Under the agreement, SRI will assist in the development of Triphase’s novel, first-in-class, fully human bi-specific antibody TRPH 011 and evaluate the role of bifunctional targeting of VEGFR-2 and TIE 2 receptors in cancer. TRPH 011 binds and neutralizes VEGFR-2/KDR and TIE 2 receptors simultaneously, resulting in sustained inhibition of tumor growth and angiogenesis (the formation of new blood vessels from pre-existing vessels and a fundamental step in the transition of tumors from a benign to a malignant state).
Under the agreement, SRI will assist in the development of Triphase’s novel, first-in-class, fully human bi-specific antibody TRPH 011 and evaluate the role of bifunctional targeting of VEGFR-2 and TIE 2 receptors in cancer. TRPH 011 binds and neutralizes VEGFR-2/KDR and TIE 2 receptors simultaneously, resulting in sustained inhibition of tumor growth and angiogenesis (the formation of new blood vessels from pre-existing vessels and a fundamental step in the transition of tumors from a benign to a malignant state).
Under the terms of the agreement, Triphase will provide funding to the laboratory of Dr. Robert S. Kerbel, senior scientist in the Biological Sciences Platform at SRI. Dr. Kerbel and his colleagues will evaluate TRPH 011 in preclinical pharmacology models. Triphase will use the findings to advance the TRPH 011 program toward an Investigational New Drug (IND) filing.