MaRS Innovation: Ahead of Innovation video launched
To coincide with our presence at the 2016 BIO International Convention (BIO) from June 6-9, 2016, MaRS Innovation launched a video explaining our role in Canada's innovation ecosystem: backing big…
To coincide with our presence at the 2016 BIO International Convention (BIO) from June 6-9, 2016, MaRS Innovation launched a video explaining our role in Canada's innovation ecosystem: backing big…
TORONTO (March 17, 2016) — QD Solar, a Canadian technology company created by the University of Toronto (U of T) and MaRS Innovation, has received $2.55 million from the Sustainable…
 On Tuesday, July 28, 2015, the federal government announced a $114-million grant to cement the University of Toronto‘s position as one of the world’s leading centres for the design and manufacture of cells, tissues and organs to treat degenerative disease.
On Tuesday, July 28, 2015, the federal government announced a $114-million grant to cement the University of Toronto‘s position as one of the world’s leading centres for the design and manufacture of cells, tissues and organs to treat degenerative disease.
This announcement was covered in The Globe & Mail and Lab Products News, and by CBC.ca, CTV News and Global TV. MaRS Innovation was specifically mentioned as a commercialization partner in the Toronto Star‘s coverage.
As the university’s commercialization agent, MaRS Innovation welcomes this news and the downstream companies and technology licenses it will create. The Honourable Ed Holder, Minister of State (Science and Technology), made the announcement.
The funding will allow U of T, in partnership with its research partners and fellow MI members — The Hospital for Sick Children, the University Health Network and Mount Sinai Hospital — to conduct transformational research and clinical translation in regenerative medicine, enhance capability in synthetic biology and computational biology and foster translation, commercialization and clinical impacts.
TORONTO (May 27, 2015) – LegWorks Inc., backed with a $2 million blend of private and Government of Canada investments catalyzed by Grand Challenges Canada, is a new Toronto-based company that will contribute to a better life for amputees in developing countries.
The LegWorks AT-knee was covered in the Toronto Star on June 1, 2015 in “Great advances being made in assistive technology” by Kate Allen and in Healio Orthotics & Prosthetics News on June 2.
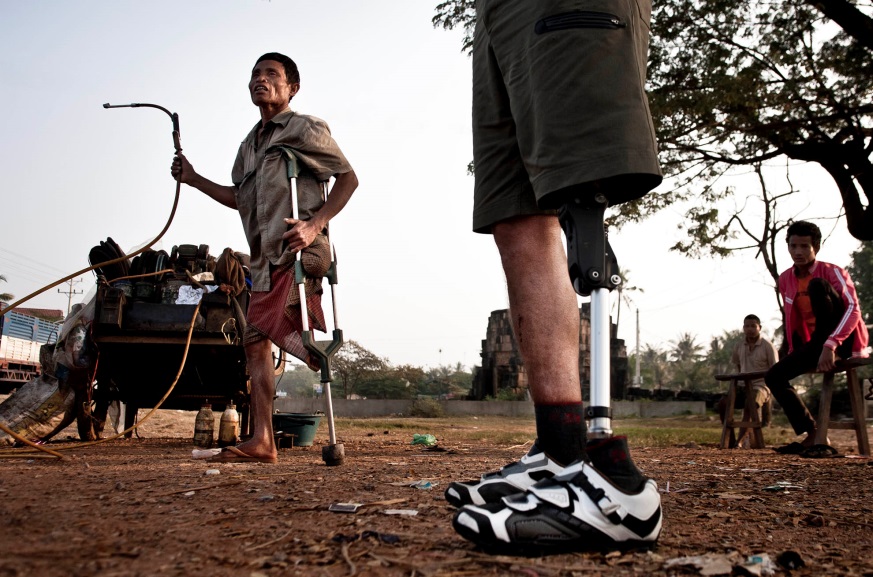
The LegWorks “All-Terrain Knee” (AT-Knee) is a safe, high-functioning, durable, affordable prosthetic knee joint developed at Toronto’s Holland Bloorview Kids Rehabilitation Hospital. It enables lower-limb amputees to walk more efficiently, safely and comfortably. Its patented design provides incredible stability, is easy to fit and maintain, and can even be used in harsh environments, including water.
“With the AT-Knee and LegWorks, it is our goal to begin to provide more universal access to better prosthetic care for individuals living with amputations around the world,” said Jan Andrysek, scientist in the Bloorview Research Institute at Holland Bloorview Kids Rehabilitation Hospital. “We want to make high-quality and well-functioning prosthetic devices affordable and accessible for the many individuals whose needs are currently left unmet.”
In trials, early users in 10 countries reported a 95 per cent preference for the relatively low-cost AT-Knee to more expensive existing technologies. Developed with a $100,000 Grand Challenges Canada seed grant awarded in 2012 to the Bloorview Research Institute, the AT-Knee easily outperformed existing technologies under rigorous conditions in El Salvador, Chile and Myanmar.
 With the new funding, LegWorks will mass produce its innovative, affordable prosthetic knee, the All-Terrain Knee (AT-Knee), the functionality and durability of which makes it ideal for amputees living in the developing world. The $2 million investment deal includes a loan via Grand Challenges Canada of up to $1 million (of which $405,000 has been dispersed), matched by MaRS Innovation, Holland Bloorview Kids Rehabilitation Hospital, Ontario Centres of Excellence and a group of private angel investors. With the $1 million expected from private investors and foundations matched by federal funds, the project will receive an anticipated $2 million to scale-up the success of the company.
With the new funding, LegWorks will mass produce its innovative, affordable prosthetic knee, the All-Terrain Knee (AT-Knee), the functionality and durability of which makes it ideal for amputees living in the developing world. The $2 million investment deal includes a loan via Grand Challenges Canada of up to $1 million (of which $405,000 has been dispersed), matched by MaRS Innovation, Holland Bloorview Kids Rehabilitation Hospital, Ontario Centres of Excellence and a group of private angel investors. With the $1 million expected from private investors and foundations matched by federal funds, the project will receive an anticipated $2 million to scale-up the success of the company.
In the first five years, LegWorks expects 37,000 units to be sold via distributors, NGOs, prosthetic clinics and government rehab facilities, in both high-income countries and the developing world.

TORONTO, March 3, 2015 — ChipCare Corporation, a University of Toronto start-up company commercializing a handheld, blood-testing platform for HIV and other infectious and non-communicable diseases has closed a $5.045 million Series A financing to bring its first-generation product to market while further developing the platform’s next generation products.
The Wall Street Journal‘s Venture Capital Dispatch blog, Yonge Street Media, BetaKit and PEHub covered this announcement, along with the University of Toronto’s news site and a follow-up BetaKit article on how smartphones and start-ups are increasing access to healthcare. Information about past ChipCare investment rounds and other company information is available in our ChipCare news archive.
Insufficient access in remote health settings to simple, accurate and affordable diagnostic tests makes it difficult to provide timely, evidence-based clinical care. Current technology within central laboratories cannot fulfill the existing need in remote health settings, including community level health facilities, remote communities, emergency departments, ICUs and doctors’ offices. The result is millions of preventable deaths from infectious and non-communicable diseases globally, reduced economic growth, and limited human development.
 ChipCare’s technology will provide simple-to-use, mobile, lab-quality blood testing in remote health settings. The company’s first HIV-related test, targeted at linking people with HIV to appropriate treatments, is scheduled to hit the market in late 2016. The company is developing other products that leverage unique attributes of ChipCare’s technology.
ChipCare’s technology will provide simple-to-use, mobile, lab-quality blood testing in remote health settings. The company’s first HIV-related test, targeted at linking people with HIV to appropriate treatments, is scheduled to hit the market in late 2016. The company is developing other products that leverage unique attributes of ChipCare’s technology.
Puffin Partners, LP, of Dallas, Texas led the financing round, which includes existing investors MaRS Innovation and Maple Leaf Angels, and new investors, including the Winfield Venture Group, Epic Capital, and additional Canadian and U.S. Angel investors.
 OTTAWA, September 16, 2013 — An innovative, handheld point-of-care analyzer, developed by ChipCare Corporation, has secured one of the largest ever angel investments in Canada’s healthcare sector.
OTTAWA, September 16, 2013 — An innovative, handheld point-of-care analyzer, developed by ChipCare Corporation, has secured one of the largest ever angel investments in Canada’s healthcare sector.
Phase II financing has closed, with an investment of $2.05M to support ChipCare’s continuing development and commercialization over the next three years.
Media coverage: Biotechnology Focus, TechVibes, BetaKit, Healthrender, Crunchbase, Toronto Star and VentureLab.
The financing evolved through a uniquely collaborative funding model among Canadian social angel investors, including Maple Leaf Angels, MaRS Innovation and the University of Toronto (Connaught Fund), with special financing leadership from Grand Challenges Canada and the Government of Canada.
Biotechnology Focus, a compendium of the Canadian life sciences industry, has published a guest column by MaRS Innovation President & CEO, Raphael Hofstein. The article explores the role of catalysis…
 TORONTO, ON (Feb. 19, 2013) – XLV Diagnostics Inc., a start-up company working to commercialize a faster, cheaper and better digital mammography technology, has received a $500,000 investment from FedNor.
TORONTO, ON (Feb. 19, 2013) – XLV Diagnostics Inc., a start-up company working to commercialize a faster, cheaper and better digital mammography technology, has received a $500,000 investment from FedNor.
Over 600 million women living in developing countries have inadequate access to breast screening for early cancer detection. In the developed world, many radiology departments are replacing traditional film and screen systems with digital technologies. In both cases, better digital mammography technology promises to solve logistical challenges and save money.
XLV’s solution has the potential to provide image quality that equals or surpasses that which is currently in use, making images easy to analyze, manipulate and transfer much like digital photographs. It will also substantially decrease the cost of digital mammography machines.
 TORONTO, February 5, 2013 — How do you make sure the brilliant ideas emerging from Toronto’s academic research community get the best possible chance to succeed?
TORONTO, February 5, 2013 — How do you make sure the brilliant ideas emerging from Toronto’s academic research community get the best possible chance to succeed?
MaRS Innovation (MI), created in 2008, bridges the chasm between these early-stage technologies and successful start-up companies and licensable technologies. By offering early-stage funding in tandem with hands-on management, mentorship and IP strategy protection, MI acts as a commercialization agent for its 16 member institutions.
This announcement was covered by TechVibes, Yonge Street Media and CanTech Letter.
The Networks of Centres of Excellence (NCE) has recognized the increasing strength of this novel partnership by awarding MI $14.95 million in funding through the Centres of Excellence for Commercialization and Research (CECR) program.
TORONTO, May 9, 2011 – York University has become the latest member of MaRS Innovation, the commercialization agent for many leading Toronto-based universities, hospitals and research institutes.

“York has become one of Canada’s fastest-growing centres for research and innovation,” said Stan Shapson, vice-president Research & Innovation at York University. “We typically get 10 to 20 discovery disclosures a year. Joining MaRS Innovation allows us to deliver the most competitive commercialization services to the researchers making these discoveries. We’re confident that membership in MaRS Innovation will boost that number and accelerate the commercialization of York’s most promising research.”
Earlier this year, York University launched its Innovation York office. Based in York Region, Innovation York works with other partner organizations in the Markham Convergence Centre to build upon research partnerships between York researchers and life science and technology companies based in York Region and the Greater Toronto Area. It’s also making York’s research and infrastructure more accessible to industry, government agencies and community partners.