Sunnybrook forms new industry partnership to advance WaveCheck technology
Co-development agreement, brokered by MaRS Innovation, to advance ultrasound chemotherapy monitoring technology as clinical tool
 TORONTO (March 12, 2015) — Sunnybrook Health Sciences Centre and MaRS Innovation today announced a co-development agreement for WaveCheck, an ultrasound technology that transforms conventional equipment so that physicians can monitor a breast cancer tumour’s response to chemotherapy.
TORONTO (March 12, 2015) — Sunnybrook Health Sciences Centre and MaRS Innovation today announced a co-development agreement for WaveCheck, an ultrasound technology that transforms conventional equipment so that physicians can monitor a breast cancer tumour’s response to chemotherapy.
This announcement was covered in Biotechnology Focus, Aunt Minnie.com (radiology blog), dotmed.com, Bio-Medicine.org, Bloomberg Business and Research Views.
The partnership with GE Healthcare, brokered by MaRS Innovation, seeks to develop WaveCheck as a clinical tool that gives clinicians rapid, improved transparency to determine if breast cancer tumors are responding to chemotherapy.
WaveCheck is a clinical technique invented, refined and tested by Dr. Gregory Czarnota, chief of Radiation Oncology at Sunnybrook’s Odette Cancer Centre, and Michael C. Kolios, professor of Physics and associate dean, Research and Graduate Studies, in Ryerson University’s Faculty of Science. Their technology uses ultrasound to visually demonstrate whether chemotherapy is destroying a breast cancer tumour at the beginning of chemotherapy treatment in as little as one week. If applied to the clinic, this knowledge has the power to transform patient experience, since existing breast cancer patients typically wait until the end of treatment, anywhere from four to six months, to know if their tumor has responded.
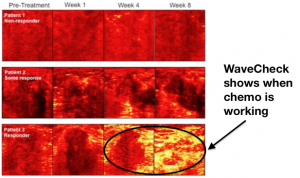
In early clinical testing, WaveCheck’s inexpensive, non-invasive, image-guided technology shows promise as an accurate, efficient way to monitor tumour response, opening the door to tailored treatment.
The agreement leverages GE Healthcare’s extensive ultrasound technology and market expertise in bringing new ultrasound innovations to global hospitals and clinics with Sunnybrook’s leadership in oncology research and cancer care through the Odette Cancer Centre.













