“Closely-held Xagenic plans to conduct beta studies this year, in advance of a major chlamydia and gonorrhea clinical trial, with its rapid X1 molecular diagnostic testing system in preparation for market launches in Europe and the U.S.,” Leonard Zahr wrote in a feature article on Xagenic’s product development progress for BioTuesdays on April 19, 2016.
“Closely-held Xagenic plans to conduct beta studies this year, in advance of a major chlamydia and gonorrhea clinical trial, with its rapid X1 molecular diagnostic testing system in preparation for market launches in Europe and the U.S.,” Leonard Zahr wrote in a feature article on Xagenic’s product development progress for BioTuesdays on April 19, 2016.
Xagenic was founded by University of Toronto Professor Shana Kelley in partnership with MaRS Innovation and U of T’s Innovations & Partnerships Office in 2010. The company has raised $52.7 million (Canadian) to develop its X1 platform for eventual use in doctors’ offices.
The full piece, “Xagenic readies X1 molecular diagnostic beta trials,” is available on BioTuesdays‘ website. Here’s an excerpt.
“The Xagenic X1 platform is a revolutionary diagnostic system that allows the user to perform lab-quality molecular assays in a physician’s office or other clinical care setting, with a time-to-result of 20 minutes or less,” Ihor Boszko, VP of business development, says in an interview with BioTuesdays.com. “This system is poised to transform the way healthcare is delivered.”
Mr. Boszko explains that the X1 instrument has been carefully designed to meet the requirements for use in a physician’s office, including its compact size, affordable pricing and ease of use.
“We are initially targeting physicians’ offices with our panel for chlamydia and gonorrhea,” he says, noting that the platform is user-friendly, with minimal training and no lab skills required.
Mr. Boszko says a rapid diagnosis will allow physicians to prescribe antibiotics, if necessary, before a patient leaves the office, improving patient compliance and outcomes, while reducing complications and unnecessary healthcare costs.
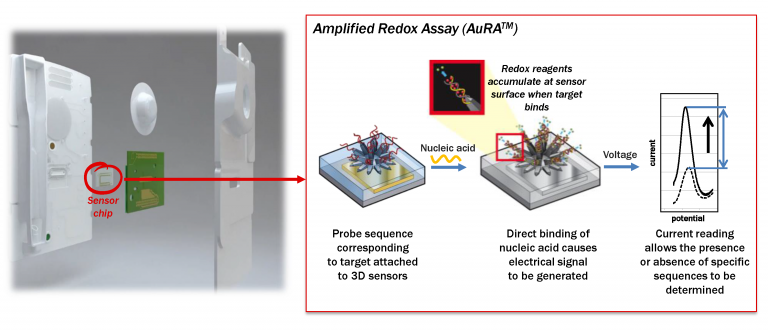
All of the reagents to perform the diagnostic test are stored on the single-use cartridge, which contains a proprietary sensor chip that enables direct detection of chlamydia and gonorrhea, without the use of enzymes. This electrochemical amplification-based technology is protected by 17 patent families globally.
[…]As part of its program for commercialization, Xagenic plans to obtain performance data from third parties beta testing with the X1 platform, beginning in May this year.
Following these studies, the company plans to initiate a large clinical study for regulatory approval, with approximately 8,000 patients, comparing the X1 technology with two other FDA-cleared systems.
Mr. Boszko estimates it will take eight-to-10 months to complete the clinical study. Once concluded, the results will be used to apply for marketing approvals in Europe and the U.S., as well as for a CLIA waiver.