Bedside Clinical Systems to bring paediatric care solution to U.S. hospitals
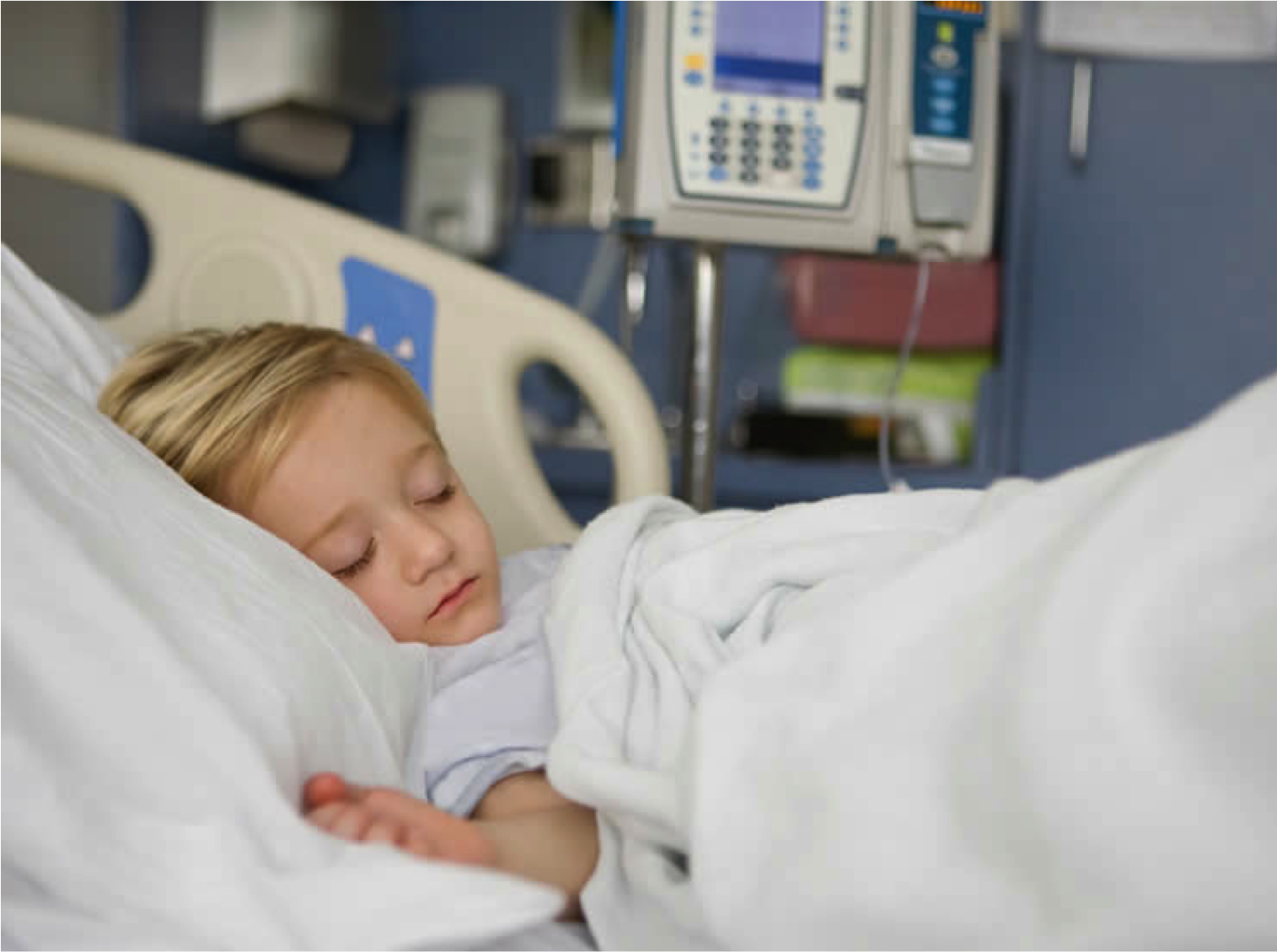
TORONTO, Canada (October 24, 2013) — Bedside Clinical Systems (BCS)’s flagship solution, Bedside Paediatric Early Warning System (BedsidePEWSTM), has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). For the complete BedsidePEWS™ FDA Medical Device listing (K124038), click here.
This story was covered in Biotechnology Focus.
BedsidePEWSTM tracks a patient’s vital signs, such as heart rate and blood pressure, as part of routine clinical assessments and outputs a single score to indicate the child’s severity of illness. Based on this score, clinicians can mitigate the risk of cardiopulmonary arrest by taking preventive action before or as a child patient’s health begins to deteriorate.
Beyond the clear benefits of preventing unexpected code blue-related deaths in individual patients, by detecting clinical deterioration early BedsidePEWSTM also reduces intra-hospital patient transfers (e.g., admission to critical care and ICU) and the need for rehabilitation from code blue incidents.

“FDA 510(k) approval allows BCS to bring our solution to U.S. hospitals and help prevent cardiopulmonary arrest in hospitalized children,” says Rajesh Sharma, BCS’ president and chief marketing officer. “With this proven system, we will positively impact the quality and safety of care provided to children, resulting in improved health outcomes. Our vision is to improve paediatric care everywhere: as the only FDA-cleared system, BedsidePEWS will benefit patients, families, and healthcare providers and payers.”
The BedsidePEWSTM technology is built on several clinical studies led by Dr. Christopher Parshuram, a paediatric physician and senior scientist at Toronto’s Hospital for Sick Children. Parshuram’s research on the BedsidePEWSTM methodology was validated through a five-year clinical study across four university-affiliated hospitals, demonstrating that BedsidePEWSTM identified patients at risk of a code blue with at least one hour’s notice.
“MaRS Innovation is proud to partner with The Hospital for Sick Children and provide business development and commercialization services to its researchers,” says Dr. Raphael Hofstein, president and CEO. “BCS is one of our earlier start-ups and we are proud and gratified to see their flagship product achieve FDA 501(k) approvals, which demonstrates both the rigour of its science and strength of its intellectual property. BCS is now well positioned to accelerate the pace of healthcare improvements emerging from SickKids in Canada and around the world.”
BCS is excited to demonstrate BedsidePEWSTM at the American Academy of Pediatrics conference (www.aapexperience.org) in Orlando, Florida from October 26 to 29, 2013.
About Bedside Clinical Systems
Bedside Clinical Systems (BCS) is a start-up company, created in partnership by The Hospital for Sick Children (SickKids) and MaRS Innovation, which translates and deploys paediatric best practices developed through decades of research at SickKids and the University of Toronto.

